Falcon R&D Program
As the Falcon bird is known to be an intelligent creature with unprecedented senses and skills, our R&D program is uniquely equipped to unveil new, detailed insights in the genomic, biologic and clinical nature of dermatologic diseases.
Under the wings of the Falcon R&D Program, a series of specific studies and projects is initiated, aimed at developing and introducing an array of diagnostic utilities, to provide physicians with the tools to optimize the clinical pathway of their patients.
These diagnostic utilities are all based on gene expression signatures.
Pipeline
In our R&D centered partnerships with clinicians and academia, entrepreneurial drive interacts with scientific
expertise to tackle unmet needs in personalized healthcare.
The Falcon R&D Program pipeline includes multiple study initiatives on dermatologic diseases,
such as Melanoma and cutaneous Squamous Cell Carcinoma.
The following table is subject to ongoing updates as the study initiatives evolve.
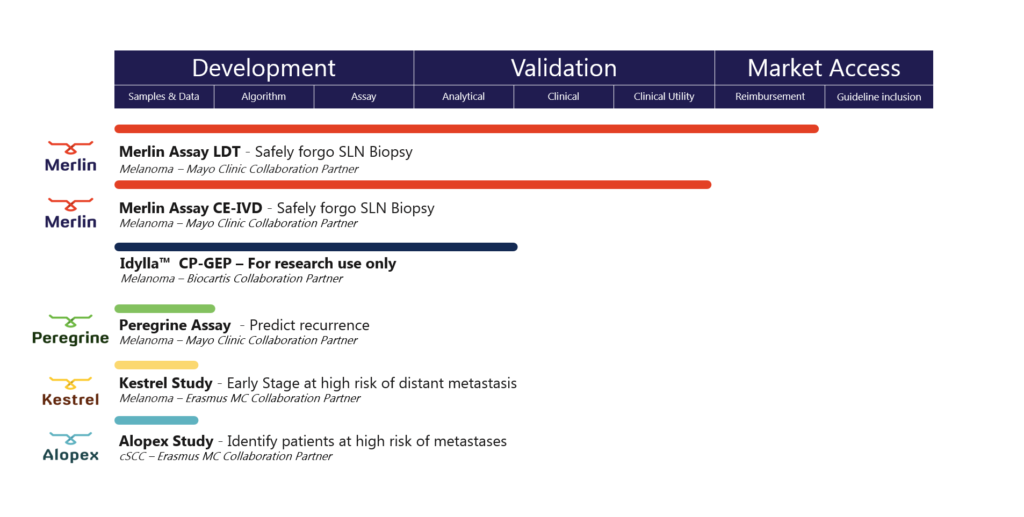

Falcon’s Study Initiatives
Merlin Study Initiative
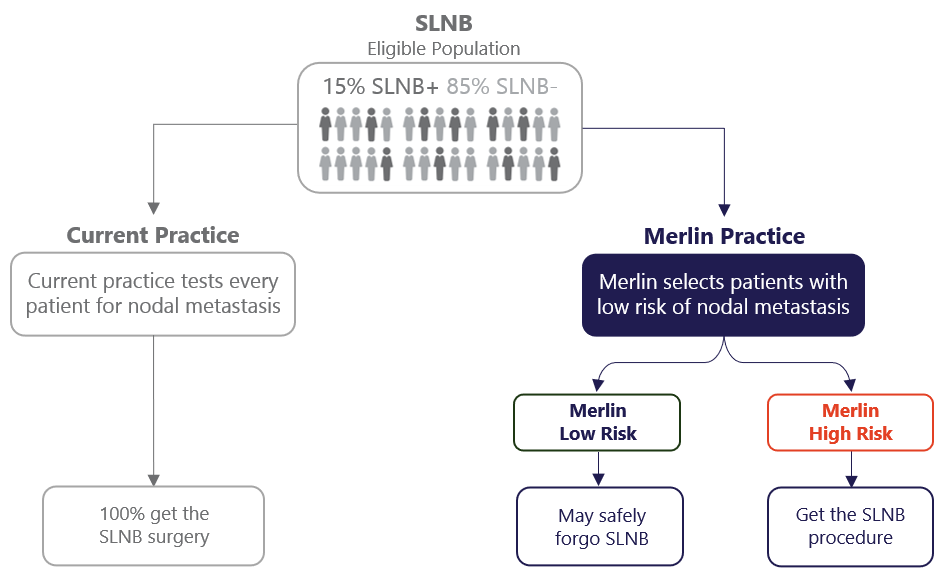
The Merlin Study Initiative helps answering the question “Can this patient safely forgo a Sentinel Lymph Node Biopsy?”. At the core of this initiative is a proprietary algorithm, called the CP-GEP model. CP-GEP interrogates 8 genes of interest, directly correlated to risk of nodal metastasis, and integrates clinicopathologic variables (patient age and Breslow thickness) into the analysis. The model is able to predict a melanoma patient’s nodal metastatic risk without an invasive Sentinel Lymph Node Biopsy surgery. Ongoing extensive validation studies in the US and EU have confirmed that the test accurately stratifies low- and high-risk patients.


Peregrine Study Initiative
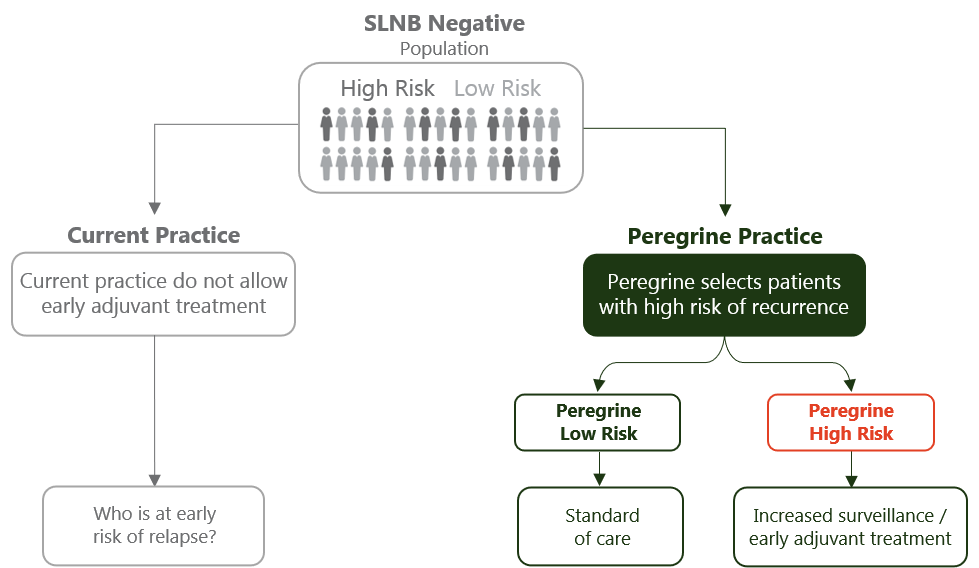
The Peregrine Study Initiative helps answering the question “Which patient is at high risk of disease recurrence?”. These patients may benefit from increased surveillance or early adjuvant therapy.
The initiative investigates clinical decision making by assessing individual risk profiles for recurrence, despite having a negative sentinel lymph node in patients with thin and intermediate thickness melanomas (Breslow thickness 0.8-4mm).
Kestrel Study Initiative
The Kestrel Study Initiative aims at investigating patients with early-stage melanoma at high risk of distant metastasis.
Approximately 90% of all melanoma patients are diagnosed with an early-stage melanoma which is thin and has not spread to the lymph nodes at diagnosis. However, 41% of melanoma-related deaths occur among these early-stage patients that initially have a good prognosis. As current predictors are not sufficient to identify this specific patient group, the consortium will dedicate its research to find novel variables (e.g. genes and proteins in the primary tumor) and attempt to develop accurate biomarkers for this specific purpose.




Alopex Study Initiative
The Alopex Study Initiative aims at the discovery and validation of a model that identifies the cutaneous Squamous Cell Carcinoma patient at high risk of developing metastasis.
While most patients are cured by the excision of the primary tumor, 5% are at high risk of recurrence and even disease-specific death. These high-risk patients would benefit from intensive surveillance and additional treatment options. This collaboration has a unique starting point because the researchers have extensive experience and will work with the unique infrastructure of large routinely collected health care datasets of metastatic SCC patients.
Our Partners
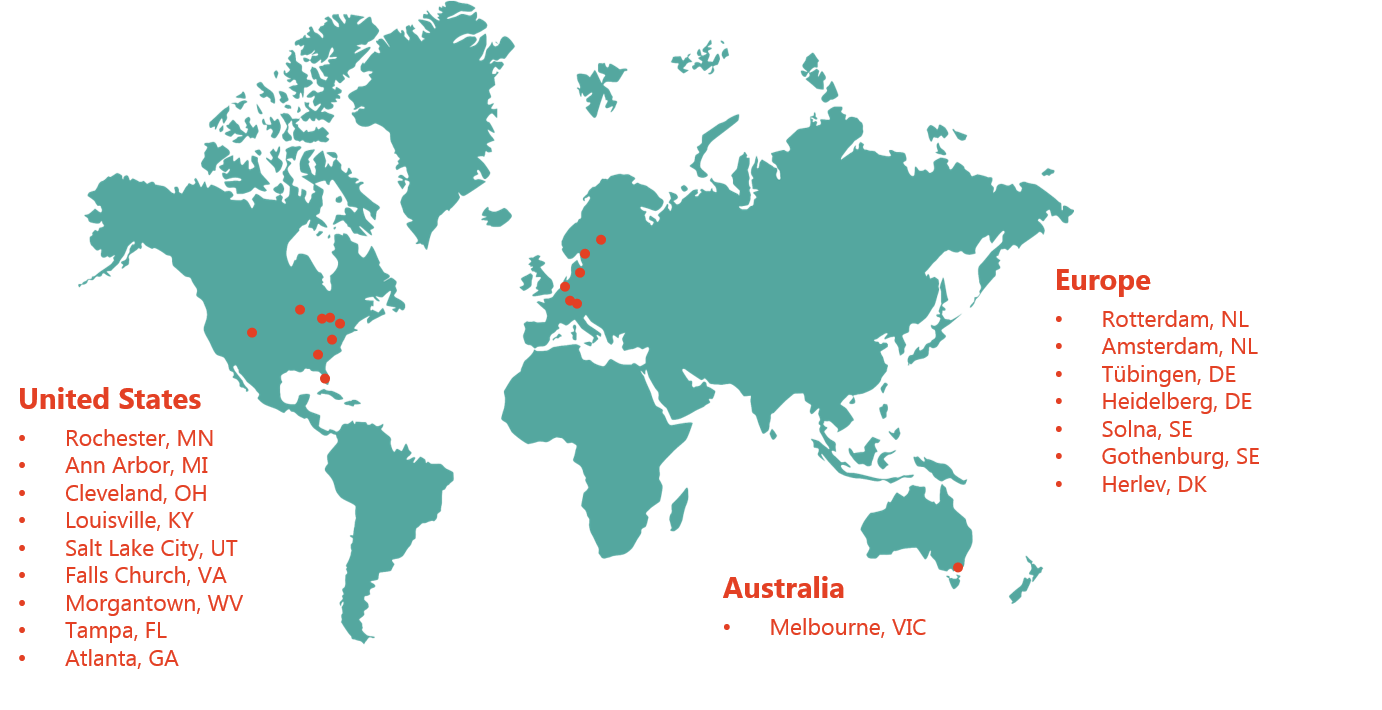
The Falcon R&D Program aims to build an extensive, global network with research institutes. Not only for research and validation studies within the existing Study Initiatives but as well to broaden the scope of the Falcon Program by starting new Study Initiatives that would entail the whole spectrum of dermatology related clinical utilities. Our extensive development and validation network are sites in Australia, Europe and in the United States.

Falcon’s scientific references
The Falcon R&D Program aims to publish its scientific and clinical research as open access peer reviewed manuscripts, available for every stakeholder in the melanoma field. Below you can find references to scientific papers, together with abstracts and posters that have been presented at – and published by (international) melanoma related conferences.
Merlin
Clinical evaluation of the clinicopathologic and gene expression profile (CP-GEP) in patients with melanoma eligible for sentinel lymph node biopsy:
A multicenter prospective Dutch study.
Stassen et al. 2023. European Journal of Surgical Oncology. Link.
Cost evaluation of the Merlin assay for predicting melanoma sentinel lymph node biopsy metastasis.
Thao et al. 2022. International Journal of Dermatology. Link.
Using the Merlin Assay for reducing sentinel lymph node biopsy complications in melanoma: a retrospective cohort study.
Hieken et al. 2022. International Journal of Dermatology. Link
Validation of a clinicopathological and gene expression profile model to identify patient with cutaneous melanoma where sentinel lymph node biopsy is unnecessary.
Johansson et al. 2021. European Journal of Surgical Oncology. Link.
Validation of CP-GEP (Merlin Assay) for predicting sentinel lymph node metastasis in primary cutaneous melanoma patients: a U.S. cohort study.
Yousaf et al. 2021. International Journal of Dermatology. Link.
Validation of a clinicopathological and gene expression profile model for sentinel lymph node metastasis in primary cutaneous melanoma.
Mulder et al. 2020. British Journal of Dermatology. Link.
Deselecting melanoma patients for sentinel lymph node biopsy during COVID-19: clinical utility of tumor molecular profiling.
Meves & Eggermont 2020. Mayo Clin Proc Inn Qual Out. Link.
Primary cutaneous melanoma risk stratification using a clinicopathologic and gene expression model: a pilot study.
Arias-Mejias et al. 2020. International Journal of Dermatology. Link.
Model combining tumor molecular and clinicopathologic risk factors predicts sentinel lymph node metastasis in primary cutaneous melanoma.
Bellomo et al. 2020. JCO Precision Oncology. Link.
Merlin
Long term outcome
Identification of stage I/II melanoma patients at high risk for recurrence using a model combining clinicopathologic factors with gene expression profiling (CP-GEP).
Amaral et al. 2022. European Journal of Cancer. Link.
Using a clinicopathologic and gene expression (CP-GEP) model to identify stage I-II melanoma patients at risk of disease relapse.
Mulder et al. 2022. Cancers. Link.
Identification of stage I/IIA melanoma patients at high risk for disease relapse using a clinicopathologic and gene expression model.
Eggermont et al. 2020. European Journal of Cancer. Link.
Alopex
Alopex Study Initiative has not yet published a peer-reviewed article.
Keep an eye on this website to follow the latest developments.
Merlin
Use of CP-GEP to identify primary cutaneous melanoma patients with low risk for SN metastasis in a prospective multicenter Dutch study during COVID-19.
Stassen et al. 2022. EADO Conference. Abstract.
Cutaneous melanoma patients with minimal SN tumor burden: CP-GEP (Merlin Assay) may guide decision-making beyond nodal assessment.
Tjien-Fooh et al. 2022. AAD Conference. Abstract.
Using the clinicopathologic and gene expression (CP-GEP) model to predict sentinel node status in patients with primary melanoma: a prospective cohort study during the COVID-19 pandemic.
Mulder et al. 2021. EADO Conference. Poster.
Independent validation study of CP-GEP model (Merlin Assay) to identify patients who can safely forgo sentinel lymph node biospy.
Johansson et al. 2021. EADO Conference. Poster.
The use of a clinicopathologic and gene expression model (Merlin Assay) to risk stratify cutaneous melanoma patients in clinical practice: A pilot study.
Bridges et al. 2020. ASDP Conference. Abstract.
A combined clinicopathologic and gene expression model (CP-GEP) identifies primary cutaneous melanoma patients who can safely forgo sentinel lymph node biopsy.
Meves et al. 2020. AAD Conference. Abstract.
Merlin
Long term outcome
Prospective multicenter evaluation (MERLIN_001 trial) of a clinicopathologic and gene expression profile test to predict sentinel node status in T1-T3 cN0 melanoma
Sondak et al., 2024. SMR Congress Abstract
CP-GEP Identifies T1a Melanoma Patients at Risk of Sentinel Lymph Node Metastasis
Yu et al., 2024. SMR Congress. Poster
Identification of patients at high risk for relapse using the Merlin Assay (CP-GEP) in an independent cohort of 432 patients with stage I/II melanoma who did not undergo sentinel lymph node biopsy
Amaral et al., 2024. ASCO conference. Poster
Using a clinicopathologic and gene expression model to predict prognosis in stage I-II primary cutaneous melanoma: a multicenter Danish cohort study.
Weitemeyer et al., 2024. EADO conference. Poster
Identification of patients at high risk for relapse by Merlin Assay (CP-GEP) in an independent cohort of melanoma patients (pts) that did not undergo sentinel lymph node biopsy: a H&N subgroup analysis.
Amaral et al., 2024. EADO conference. Abstract.
Using a clinicopathologic and gene expression (CP-GEP) model in a European cohort to identify stage II melanoma patients at high risk for disease relapse.
Mulder et al. 2021. EADO Conference. Poster.
Identification of stage IIA melanoma patients at high risk for disease relapse using a clinicopathologic and gene expression model.
Wever et al. 2020. ASCO Conference. Abstract.
Peregrine
Peregrine Study Initiative has not yet published abstracts or posters.
Keep an eye on this website to follow the latest developments.
Kestrel
Kestrel Study Initiative has not yet published abstracts or posters.
Keep an eye on this website to follow the latest developments.
Alopex
Development of a clinico-pathological model to estimate the absolute
metastasis risk in patients with cutaneous squamous cell carcinoma.
Rentroia-Pacheco et al. 2022. EADO Conference. Abstract.
